Easyhaler® Budesonide (Budesonide)
Product info | 01/12/2020
FULL PRESCRIBING INFORMATION CAN BE FOUND HERE

PRESENTATIONS:
Budesonide 100mcg, 200mcg and 400mcg
INDICATION:
For treatment of mild, moderate or severe persistant asthma for patients aged 6 and above.
CONTRAINDICATIONS:
Easyhaler Budesonide is contraindicated in patients with hypersensitivity to budesonide, lactose or milk proteins.
UNDESIRABLE EFFECTS:
The common reported adverse events are cough and throat irritation, oropharyngeal candidiasis and difficulty in swallowing.
Prescribers should consult the SmPC in relation to special warnings and precautions.

WITH EASYHALER® BUDESONIDE YOU CAN1 :
✔ Prescribe a low dose ICS for new patients with Easyhaler Budesonide (budesonide).
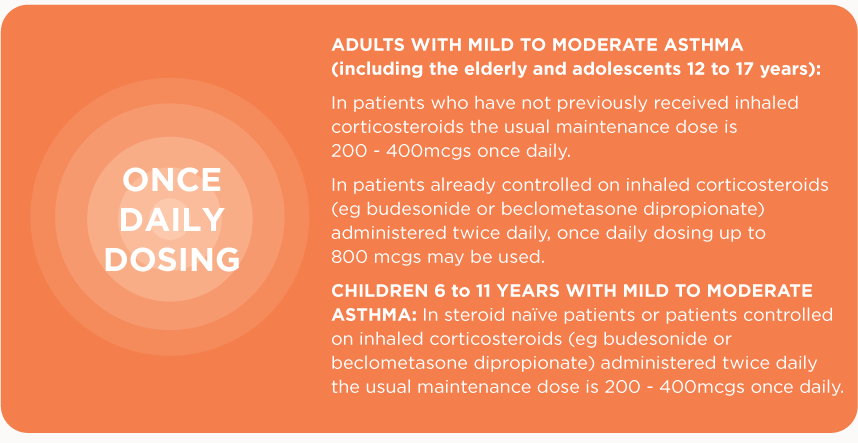
✔ Use flexible daily dosing with children and adults (see below for dosing regimes).


EASYHALER® BUDESONIDE provides you with a low carbon option
for your inhaled corticosteroid prescribing so you can:
✔ Reduce your MDI prescribing
✔ Prescribe a range of drug therapies within the Easyhaler® range and have the flexibility to move patients across the BTS / SIGN guidelines to find and maintain the lowest controlling therapy2
ESTIMATED CARBON FOOTPRINT OF DPIs and MDIs (per 200 actuations)3
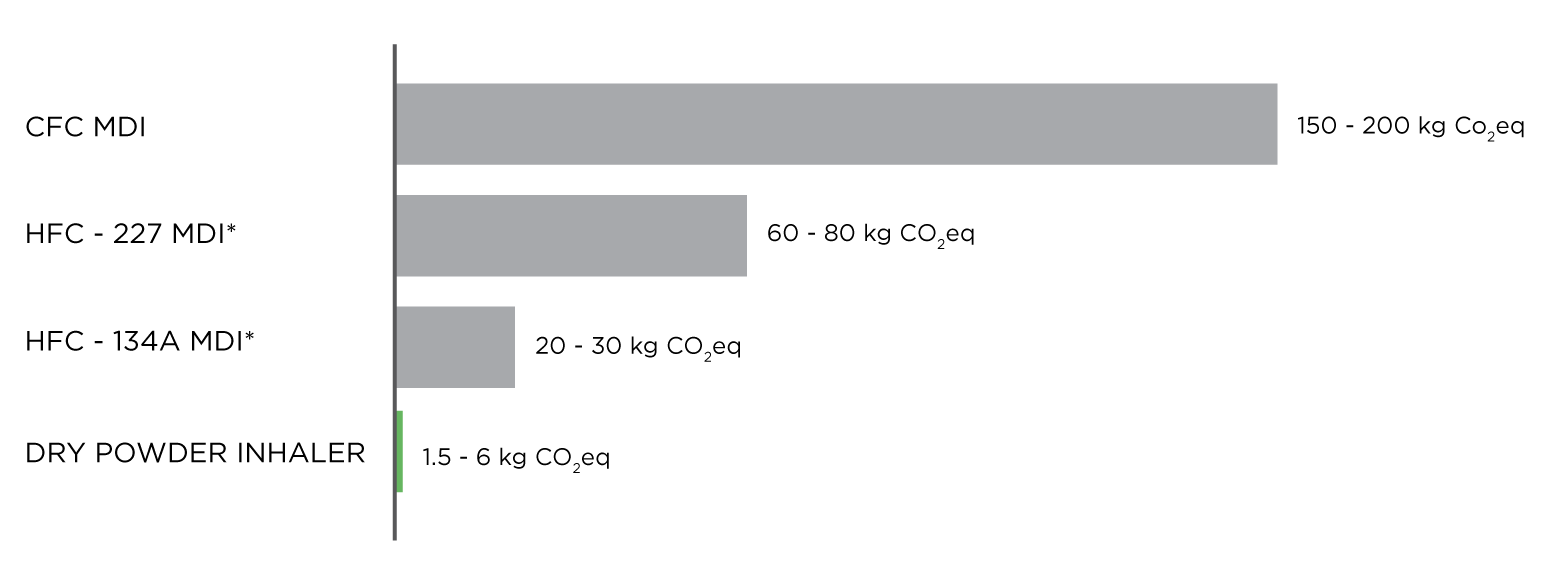
* Numbers refer to class of hydrofluorocarbon (HFC) refrigerant
Figure 1: Estimated carbon footprint of DPIs and MDIs (per 200 actuations) adapted from: Montreal protocol on substances that deplete the ozone layer. Medical and chemicals technical options committee. Assessment report 2018
BTS / SIGN = British Thoracic Society / Scottish Intercollegiate Guidelines Network, ICS = Inhaled Corticosteroids, DPI = Dry Powder Inhaler, MDI = Metered Dose Inhalers.
SmPC = Summary of Product Characteristics
Was this article helpful?
References
- Easyhaler Budesonide 100, 200, and 400 mcg. SmPC. Orion Pharma.
- BTS/SIGN. British guidelines on the Management of Asthma - a national clinical guideline. SIGN 158 Revised July 2019. https://www.britthoracic.org.uk/qualityimprovement/guidelines/asthma/
- Montreal protocol on substances that deplete the ozone layer. Medical and chemicals technical options committee. Assessment report 2018.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
May 2023 / RESP-330bbg(3)


