Fobumix Easyhaler® (budesonide / formoterol fumarate dihydrate)
Product info | 19/12/2022
FOBUMIX EASYHALER®
(budesonide/formoterol fumarate dihydrate)
Full prescribing information can be found here
 |
|
|
 |
 |

COMPOSITIONS:
- budesonide/formoterol fumarate dihydrate
STRENGTHS:
- 80/4.5mcg
- 160/4.5mcg
- 320/9mcg
METERED DOSE:
The mass of drug available within the device per actuation.
DELIVERED DOSE:
The mass of drug available within the device per actuation.
For Fobumix Easyhaler® the delivered dose and the metered dose are similar. However, for Symbicort Turbohaler® the delivered dose and the metered dose are different. For example, Symbicort Turbohaler® 100/6 and Fobumix Easyhaler® 80/4.5 deliver the equivalent dose to the patient, however, Symbicort Turbohaler® is labelled with the metered dose.
INDICATIONS:
Asthma: Maintenance Therapy - For patients aged 6 years and over
| STRENGTH | INDICATIONS |
| 80/4.5mcg | Regular treatment of asthma where the use of a combination (inhaled corticosteroid and long-acting beta-2-adrenoceptor agonist) is appropriate: patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting beta-2-adrenoceptor agonists or patients already adequately controlled on both inhaled corticosteroids and long-acting beta-2-adrenoceptor agonists |
| 160/4.5mcg* | |
| 320/9mcg* |
*Only indicated in adults and adolescents 12 years and older.
Asthma: Maintenance And Reliever Therapy (MART) - For patients aged 12 years and over 
| STRENGTH | INDICATIONS |
| 80/4.5mcg | Regular treatment of asthma where the use of a combination (inhaled corticosteroid and long-acting beta-2-adrenoceptor agonist) is appropriate: patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting beta-2-adrenoceptor agonists or patients already adequately controlled on both inhaled corticosteroids and long-acting beta-2-adrenoceptor agonists |
| 160/4.5mcg | |
| 320/9mcg | This strength is not indicated for MART |
Asthma: Reliever Therapy (as-needed, AIR therapy)
| STRENGTH | INDICATIONS |
| 160/4.5mcg | Indicated as reliever therapy for adults and adolescents 12 years and older with mild asthma. |
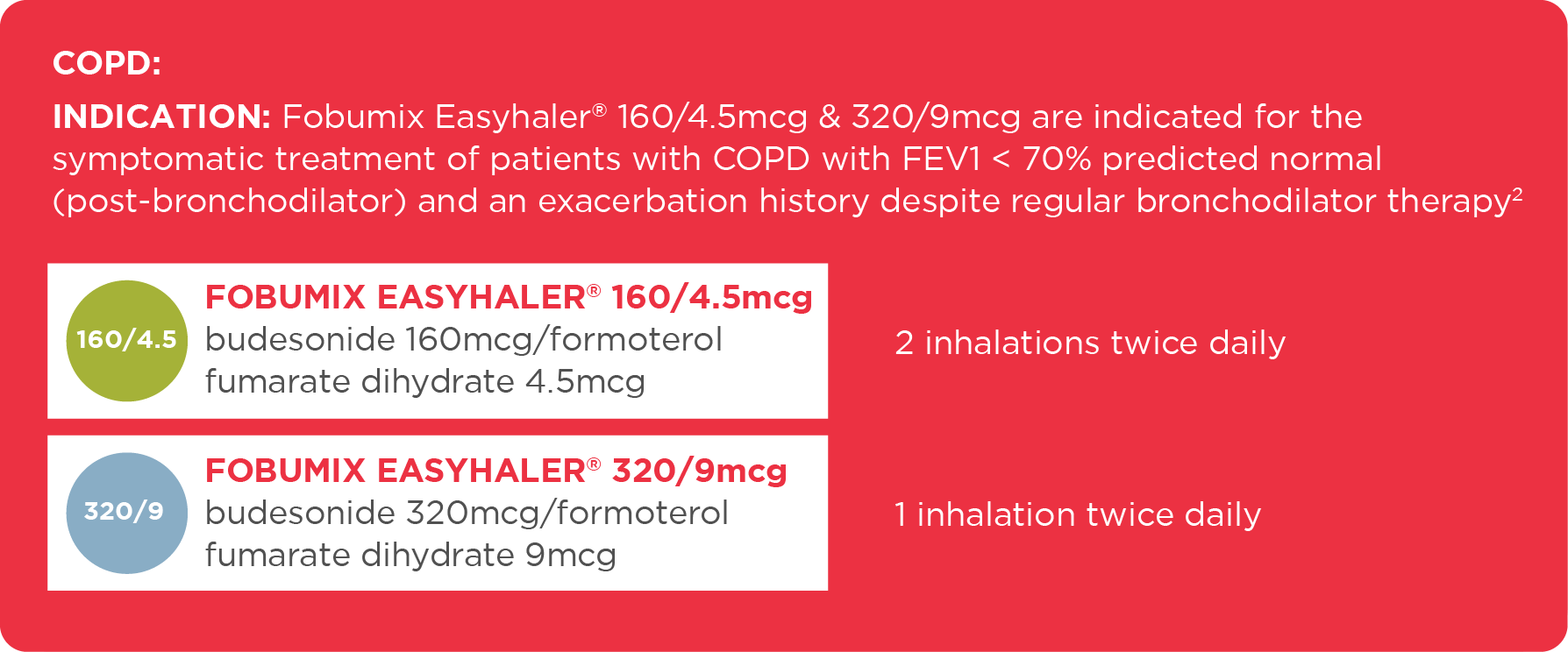
COPD - For patients aged 18 years and over
| STRENGTH | INDICATIONS |
| 80/4.5mcg | This strength is not indicated for COPD |
| 160/4.5mcg | Symptomatic treatment of adults (18 years and older) with COPD with FEV1 < 70% predicted normal (post-bronchodilators) and an exacerbation history despite regular bronchodilator therapy |
| 320/9mcg |
CONTRAINDICATIONS:
Fobumix Easyhaler® is contraindicated for patients with hypersensitivity to the active substances or lactose monohydrate (which contains milk proteins)
UNDESIRABLE EFFECTS:
The most common drug related adverse reactions are pharmacologically predictable side-effects of β2 agonist therapy, such as tremor and palpitations.
Common : Candida infections in the oropharynx, pneumonia (in COPD patients); Headache, tremor; palpitations; Mild irritation in the throat, coughing, dysphonia including hoarseness.
Prescribers should consult the SmPC in relation to special warnings and precautions.

CONSISTENT DOSING EVEN WITH LOW PATIENT INHALATION FLOW:
Fobumix Easyhaler® provides more accurate and consistent dose delivery vs Turbohaler across different patient inhalation flow rates (p<0.001, Figure 1). The dose delivery remains consistent throughout the inhaler lifespan, and is not affected by environmental stress, such as moisture, dropping, vibration, or repeated freeze-thaw cycles. Fobumix Easyhaler® ensures accurate and consistent dosing, starting from a peak inspiratory flow (PIF) of 30 L/min.2,3
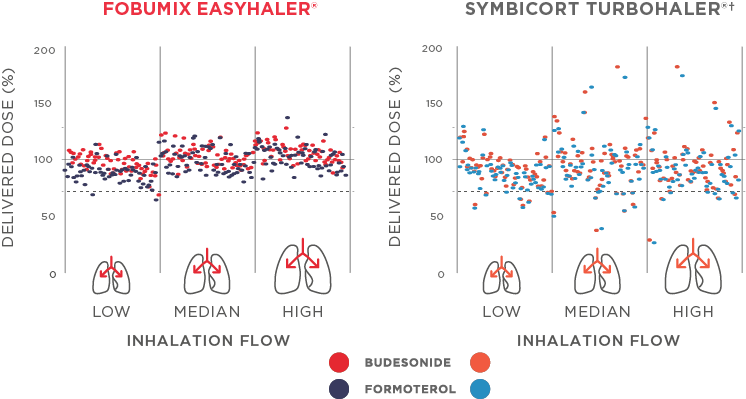
Figure 1: Adapted from Haikarainen et al., 2017. Fobumix Easyhaler® offers consistent dosing even with low patient inhalation flows


Titration to the lowest effective dose could include once daily use, when a long acting bronchodilator and inhaled corticosteroid would be required to maintain control. Children: not indicated for use in children under the age of six.2

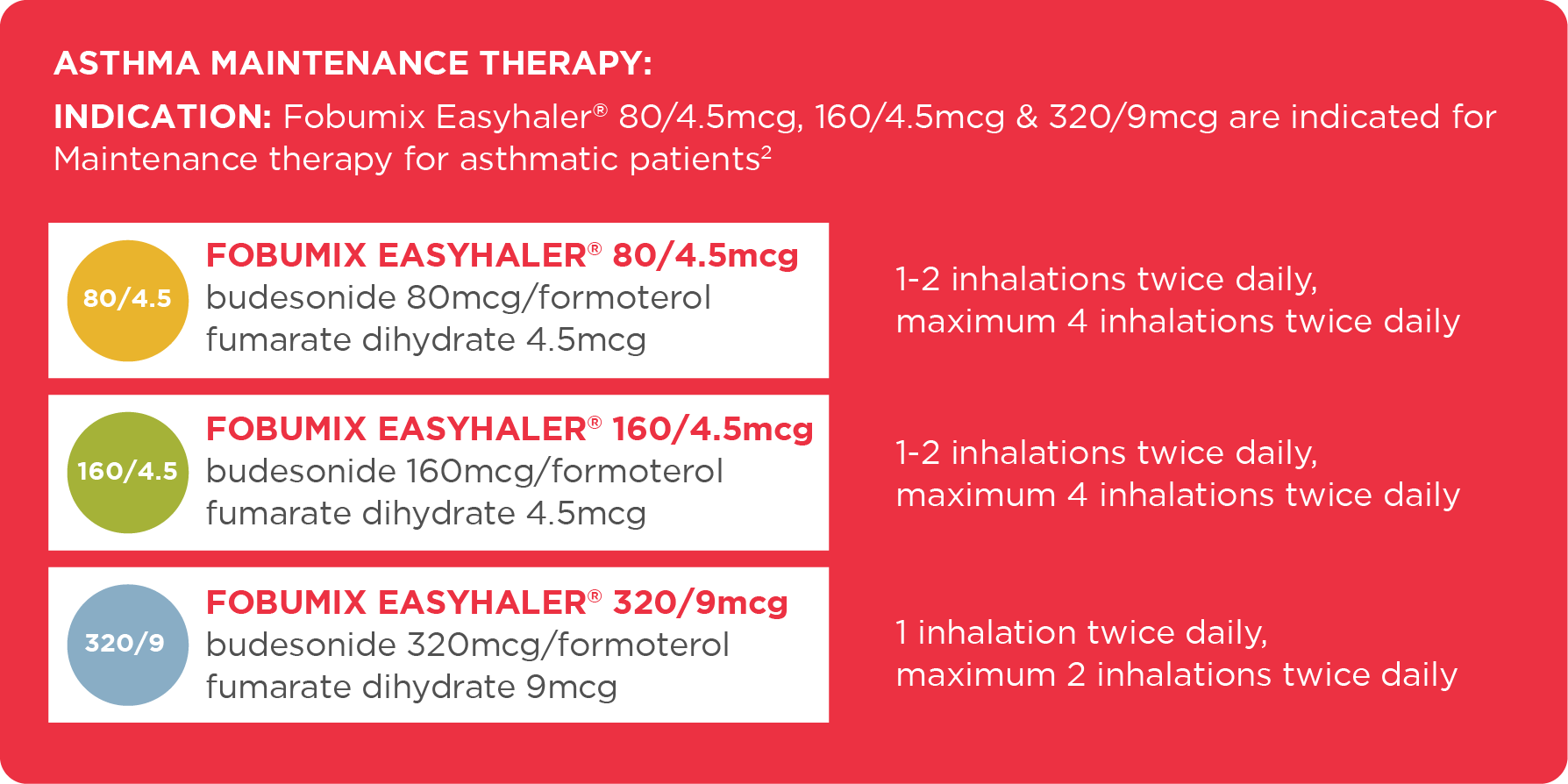
INDICATION:1
Fobumix Easyhaler® 80/4.5mcg & 160/4.5mcg are indicated for Maintenance and Reliever Therapy (MART) in asthma for patients 12 years of age and over.
Fobumix Easyhaler® 320/9 is not indicated for Maintenance and Reliever Therapy.
HOW DOES MART WORK?

Formoterol, in addition to its dose-dependent bronchodilating effect,
has a rapid onset of action of 1-3 minutes
The fast-acting formoterol in the ICS/LABA combination means patients are not required
to have an additional SABA as they can get fast acting relief with just the single inhaler.
DOSING WITH MART
USUAL DOSING:
| 80/4.5mcg | |
 |
2 inhalations per day, given as a single or divided dose + as needed inhalations |
| 160/4.5mcg | |
 |
2 inhalations per day, given as a single or divided dose + as needed inhalations |
A maximum 6 inhalations on any single occasion may be taken.
A total daily dose of more than 8 inhalations is not normally needed; however, a total daily dose of up to 12 inhalations could be used for a limited period.
For some patients on the 160/4.5 strength only, 2 maintenance inhalations twice daily may be appropriate.
Fobumix Easyhaler® 320/9 is not indicated for Maintenance and Reliever Therapy
WHAT COULD THE USUAL DOSING REGIMEN LOOK LIKE FOR A PATIENT?

A hypothetical example illustrating a possible dosing regimen for a patient using Fobumix Easyhaler® 80/4.5mcg or 160/4.5mcg. The example demonstrates a patient taking their Fobumix Easyhaler® twice per day as a divided dose for their maintenance treatment, as well as also taking additional inhalations of their Fobumix Easyhaler® as needed throughout the day to relieve their symptoms.
COMPREHENSIVE CARE – IMPROVED ASTHMA CONTROL
Fobumix Easyhaler® combines both maintenance and reliever therapy (MART) within a single inhaler device. Patients treated with budesonide/formoterol as part of a MART regimen experience less asthma exacerbations compared to those receiving fixed-dose therapy in individual devices with as needed terbutaline or formoterol (p<0.0001).2-6

EXPERIENCING LIFE WITH FEWER SYMPTOMS
Fobumix Easyhaler® significantly improves asthma and COPD-related quality of life (mini-AQLQ, mMRC dyspnea scale, p<0.001).
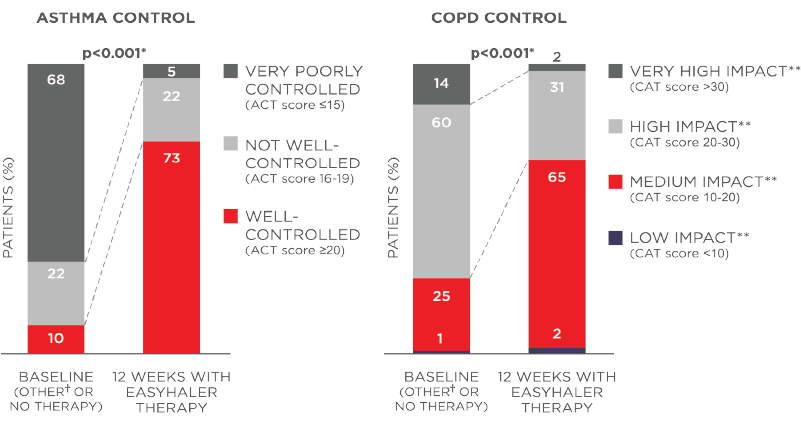
Figure 2: Adapted from Tamási et al., 2018. Fobumix Easyhaler® combination therapy improves asthma and COPD control. N (total) = 1498 patients (621 with asthma, 778 with COPD, and 99 with asthma-COPD overlap; 455 newly diagnosed and 1043 patients switching from another inhaler device). *P<0.001 for average ACT and CAT score.
**Impact of COPD symptoms on everyday life. Patients received 12 weeks of Fobumix Easyhaler therapy.6
†This article refers to UK brand names. Within the clinical papers the brand names reflect the location of where the studies took place.
COPD = Chronic obstructive pulmonary disease, ICS = Inhaled corticosteroid, SABA = Short acting beta agonist, LABA = Long acting beta agonist, ICS + LABA = Inhaled corticosteroid + long acting beta agonist.
References
- Carbon Neutral Product Certificate for Easyhaler® product range. Available on request from Orion Pharma.
- Fobumix Easyhaler® 80/4.5, 160/4.5, and 320/9 mcg. SmPC. Orion Pharma.
- Haikarainen J, Selroos O, Löytänä T, Metsärinne S, Happonen A, Rytila P. Budesonide/Formoterol Easyhaler®: Performance under simulated real-life conditions. Pulm Ther 2017;3:125–38.
- Ghosh S, Ohar J, Drummond M. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Pulm Drug Deliv 2017;30(6):381–87.
- Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract, May 2007,61,5,725–736.
- Tamási L, Szilasi M, Gálffy G. Clinical effectiveness of budesonide/formoterol fumarate Easyhaler® for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes. Adv Ther 2018;35(8):1140–52.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
March 2025/RESP-330bba(5)




