Easyhaler® in Asthma Guidelines
Product info | 01/12/2020
Prescribing Information and Adverse Event Reporting can be found at the bottom of this page.
ONE DEVICE AT EVERY STEP OF THE BTS/NICE/SIGN 2024
ASTHMA GUIDELINES1
WITH THE EASYHALER® RANGE, YOU CAN:
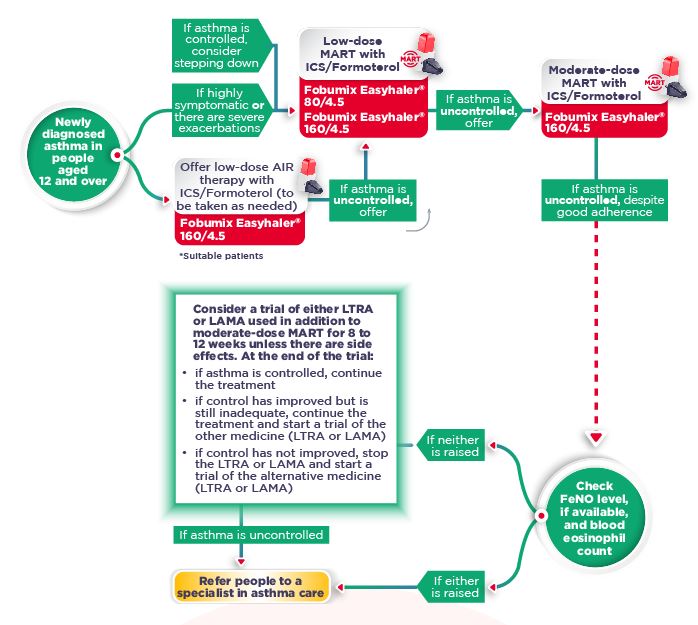
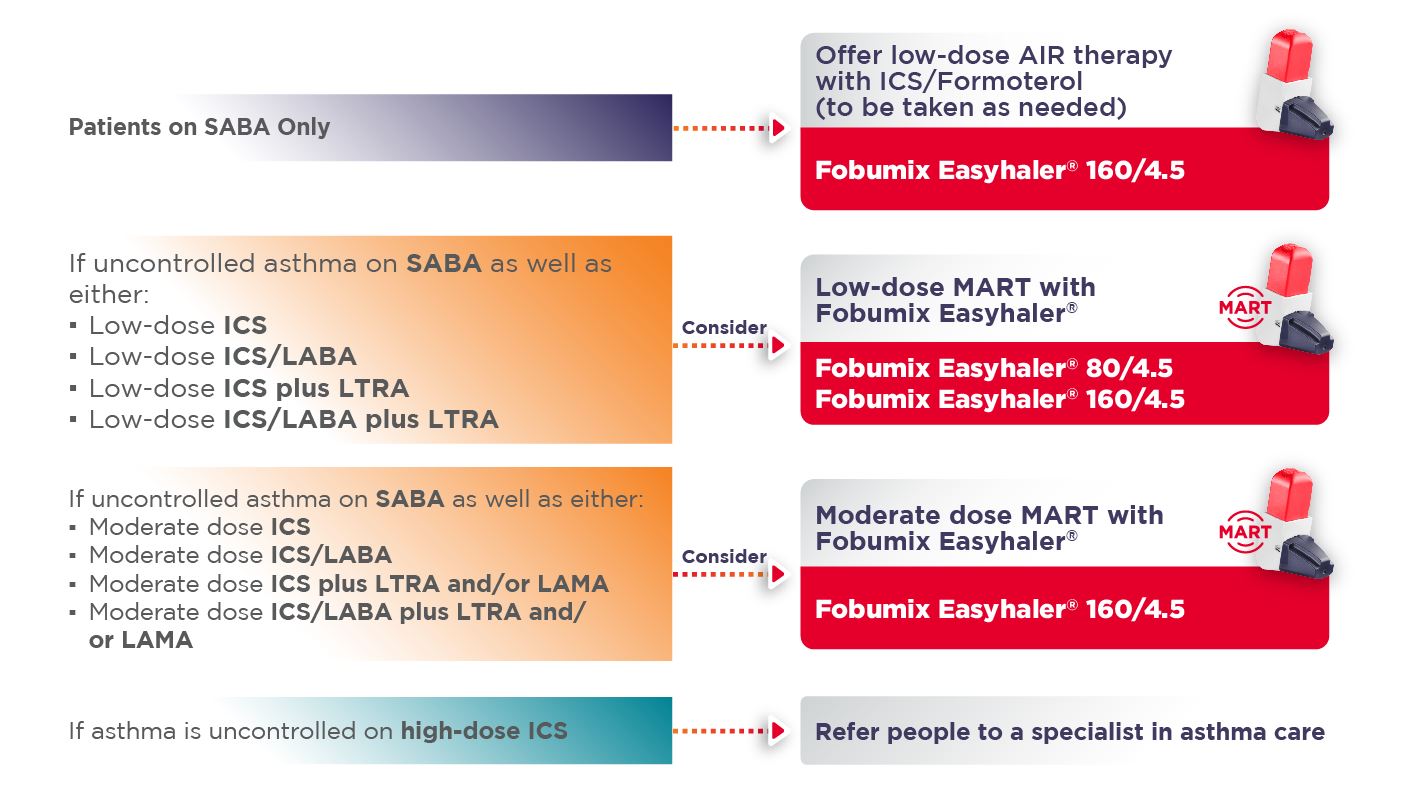
- Prescribe Fobumix Easyhaler® (budesonide/formoterol fumarate dihydrate) 160/4.5mcg for reliever therapy (as-needed, AIR), MART and maintenance therapy in patients 12 years and above2
- Prescribe twice-daily paediatric low dose ICS for new patients (aged 6 years and above) with Easyhaler® Budesonide (budesonide) with a short-acting beta2 agonist (SABA) with Easyhaler® Salbutamol (salbutamol sulfate)3,4
- The Easyhaler® range provides the flexibility to move patients across the BTS/NICE/SIGN 2024 guidelines to find and maintain the lowest controlling therapy1
Download your "Fobumix Easyhaler® and the Guidelines" to discover where Fobumix Easyhaler® sits in the latest BTS/NICE/SIGN 2024 guidelines.
CLICK HERE for full BTS/NICE/SIGN Guidance (NG245) |
Easyhaler® Budesonide Indication: Treatment of mild, moderate or severe persistent asthma. Note: Easyhaler® Budesonide is not suitable for the treatment of acute asthma attacks.
Fobumix Easyhaler® Indication:
Asthma: Regular treatment of asthma where the use of a combination (inhaled corticosteroid and long-acting beta-2-adrenoceptor agonist) is appropriate: patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting beta-2-adrenoceptor agonists or patients already adequately controlled on both inhaled corticosteroids and long-acting beta-2-adrenoceptor agonists.
Fobumix Easyhaler® 80/4.5mcg: indicated for maintenance therapy in patients ≥6 years of age, and maintenance and reliever therapy (MART) in patients ≥12 years of age.
Fobumix Easyhaler® 160/4.5mcg: indicated for reliever therapy (as-needed, AIR therapy) in mild asthma, maintenance therapy and MART in patients ≥12 years of age.
Fobumix Easyhaler® 320/9mcg: indicated for maintenance therapy only in patients ≥12 years of age.
Note: Fobumix Easyhaler® 80/4.5mcg is not appropriate in patients with severe asthma.
Easyhaler® Salbutamol Indication: Symptomatic treatment of asthma attacks and exacerbations of asthma in adults and children aged 4 years and over; prevention of exercise-induced bronchospasm or before known unavoidable allergen exposure; symptomatic treatment of broncho-asthma and other conditions associated with reversible airways obstruction.
Was this article helpful?
REFERENCES:
- BTS/NICE/SIGN Guidelines 2024. Asthma: diagnosis, monitoring and chronic asthma management. Revised November 2024. https://www.nice.org.uk/guidance/ng245. April 2025.
- Fobumix Easyhaler® SmPC, 80/4.5mcg, 160/4.5mcg, 320/9mcg. Orion Pharma.
- Easyhaler® Budesonide SmpC 100mcg, 200mcg, 400mcg. Orion Pharma.
- Easyhaler® Salbutamol, 100mcg, 200mcg. Orion Pharma.
BTS/NICE/SIGN = British Thoracic Society / National Institute for Health and care Excellence / Scottish Intercollegiate Guidelines Network
ICS = Inhaled Corticosteroids, SmPC = Summary of Product Characteristics
DPI = Dry powder inhalers
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd
on 01635 520300.
May 2025/RESP-330bbf(4)