What is MART?
Maintenance And Reliever Therapy (MART)
Product info |
22/04/2020

Fobumix Easyhaler®
budesonide/formoterol fumarate dihydrate
Prescribing information and Adverse event reporting can be found at the bottom of this page.
WHAT IS MAINTENANCE AND RELIEVER THERAPY (MART)?
MART is a treatment regimen where both the Inhaled Corticosteroid (ICS) and the beta2 agonist are combined into a single inhaler and are used for both maintenance therapy and during acute exacerbations. The use of a MART regimen is only available for ICS/LABA combinations where the LABA has a fast-acting component.1
As per the latest BTS/NICE/SIGN chronic asthma management guidelines (NG245), MART is now recommended within the treatment pathway for patients 12 years and over - please see the full guideline for further information, click here.2
HOW DOES MART WORK?
MART regimen have two primary purposes. The steroid used in the combination, i.e. Budesonide, is used to prevent asthma exacerbations. The Long Acting Beta2 Agonist (LABA) i.e. Formoterol, is used to relieve symptoms during mild exacerbations. Due to the combination of ICS/LABA medicines, patients are not necessarily required to have an additional SABA as they can get fast acting relief with just the single combination inhaler.1
| ● | Preventer (ICS) Budesonide is a glucocorticosteroid that when inhaled has a dose-dependent anti-inflammatory action in the airways, resulting in reduced symptoms and fewer asthma exacerbations.3 |
● |
Reliever (LABA) Formoterol when inhaled results in rapid and long-acting relaxation of bronchial smooth muscle in patients with reversible airways obstruction. The bronchodilating effect is dose-dependant, with an onset of effect within 1-3 minutes. The rapid onset of effect induced by formoterol means patients can calm their symptoms quickly should they become wheezy or breathless. The duration of effect is at least 12 hours after a single dose.4 |
WHAT DOES A MART DOSING REGIMEN LOOK LIKE WITH FOBUMIX EASYHALER®? (BUDESONIDE/FORMOTEROL FUMARATE DIHYDRATE)
 |
|
|||||||||||||||
Fobumix Easyhaler® 80/4.5 & 160/4.5 are indicated for MART therapy for patients 12 years and above with:
- inadequate asthma control and in frequent need of reliever medication
- prior asthma exacerbations requiring medical intervention.5
Click here for further information on Fobumix Easyhaler® and MART prescribing.
WHAT DIFFERENCE WILL A MART REGIMEN MAKE TO MY INHALER PRESCRIBING?
A MART regimen using Fobumix Easyhaler should only be used in patients 12 years and above. For most patients, you will only need to prescribe one inhaler. This is because the reliever part of the medicine is covered with the use of formoterol as a LABA meaning that should an asthma exacerbation occur, patients can use their budesonide/formoterol combination to help alleviate their symptoms.4
WHAT EVIDENCE IS AVAILABLE FOR MART AS AN EFFECTIVE TREATMENT FOR ASTHMA?
A study by Kuna et al., (2007) looked to see whether budesonide/formoterol used as a Maintenance & Reliever Therapy (MART) could be as effective at reducing severe exacerbations as high dose fixed dose combination inhalers.5
The randomised, double blind 6 month study with 3335 symptomatic adults and adolescents split the trial into three arms to compare the efficacy of MART:
| ● | A) | FIXED DOSE THERAPY - Salmeterol/fluticasone combo + SABA (terbutaline) – 25/125mcg two inhalations bid + as needed SABA |
| ● | B) | FIXED DOSE THERAPY - Budesonide/formoterol combo + SABA (terbutaline) – 320/9mcg one inhalation bid + as needed SABA |
| ● | C) | MART - Budesonide/formoterol for MART – 160/4.5mcg – One inhalation bid + as needed inhalations |
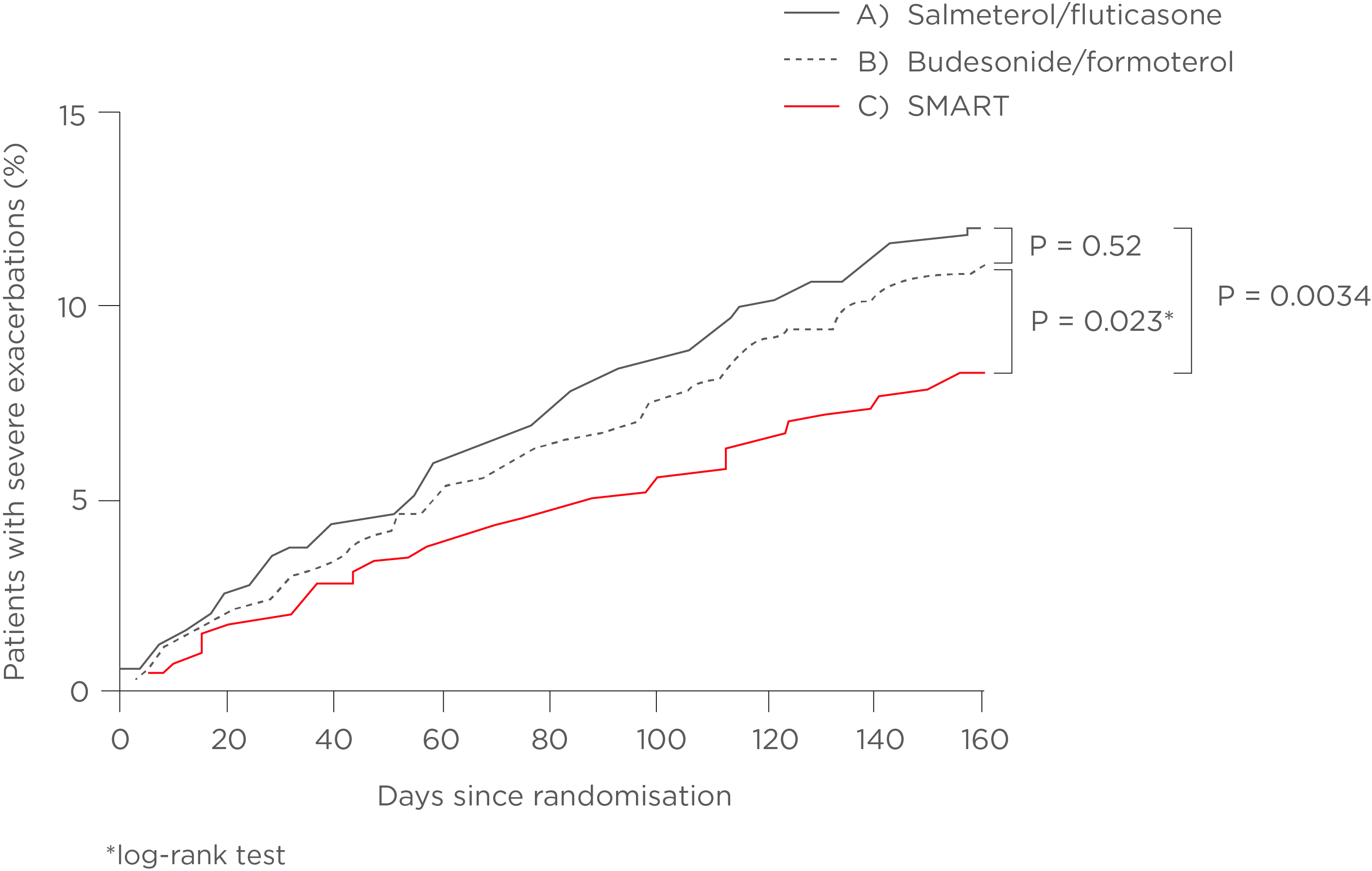
Figure 1: Adapted from Kuna et al., 2007. The results showed the number of severe asthma exacerbations was reduced by 39% (p < 0.001) in the MART group compared with fixed-dose salmeterol/fluticasone and by 28% (p= 0.0048) compared with fixed-dose budesonide/formoterol. The total number of exacerbations was similar in the two fixed-dose groups.
Furthermore budesonide/formoterol as a MART regime prolonged the time it took for patients to experience their first exacerbation requiring hospitalisation, emergency room treatment or oral steroid treatment, as demonstrated in Figure 1.5
WHAT IMPACT DOES A MART REGIMEN HAVE ON OVERALL TREATMENT LOAD OF AN ASTHMA PATIENT?
The National review of asthma deaths (NRAD) report highlighted the need for prevention of asthma using ICS.6 However, it is well known that there are associated risks with the overuse of steroid derived medications including immune and renal supression.8
Different inhaled cortocisteroids require varied levels of potency in order to have equal efficacy therefore, in order to compare the treatment groups, all ICS doses used across the three treatment groups were converted to the equivalent ICS dose of beclomethasone dipropionate (BDP).5
The mean doses of ICS for the three groups is demonstrated below:
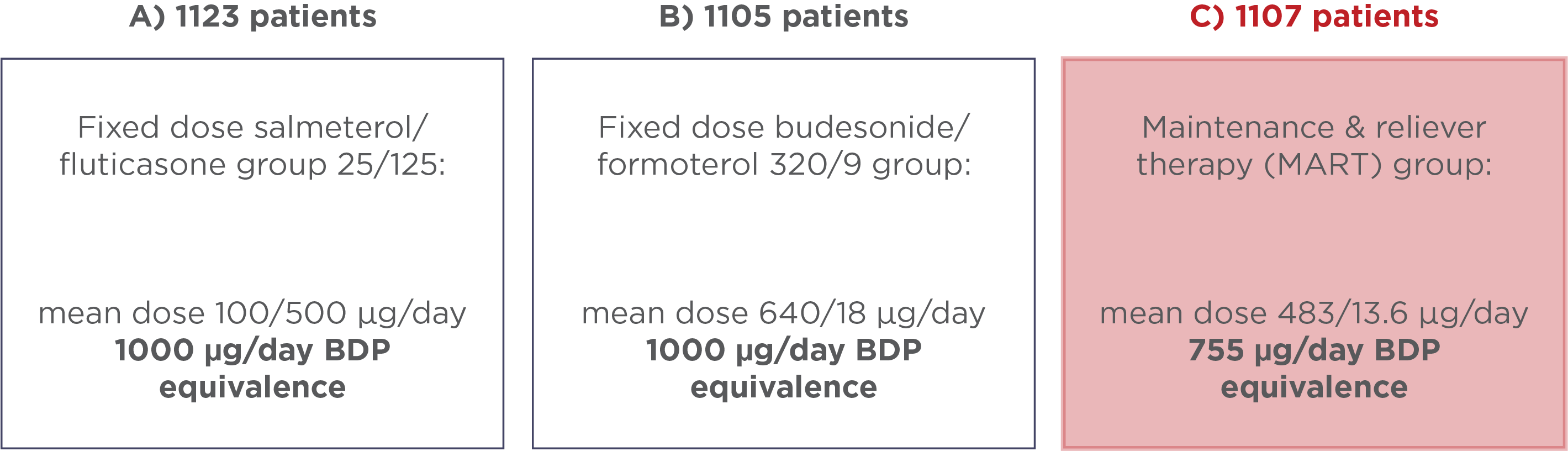
Figure 2: Mean ICS doses converted to BDP equivalents based on GINA guidelines across the three treatment groups used in Kuna et al., 2007.6
The study demonstrated that using budesonide/formoterol in a MART regime reduced the risk and rate of severe exacerbations compared with higher maintenance dose of fixed dose budesonide/formoterol. It achieves this improvement at average daily drug load with the level of ICS recorded reducing by 25% vs fixed dose budesonide/formoterol.6
SUMMARY
By using a budesonide/formoterol combination as part of a MART regimen, your patients may be able to:
- Experience less asthma exacerbations that require hospital treatment vs fixed dose combination
budesonide/formoterol + SABA (terbutaline)6 - Reduce the level of steroid patients are administering on a daily basis vs fixed dose combination
inhaler of budesonide/formoterol + SABA (terbutaline)6 - Achieve better adherence by having a single inhaler as both their preventer and reliever vs fixed
dose budesonide/formoterol + SABA (terbutaline)6
Further information on Fobumix Easyhaler® can be found here.
References
- Di Marco F. (2020). Today’s improvement in asthma treatment: role of MART and Easyhaler®. Multidisciplinary Respiratory Medicine, 15(1), 649. https://doi.org/10.4081/mrm.2020.649
- BTS/NICE/SIGN Guidelines 2024. Asthma: diagnosis, monitoring and chronic asthma management. Revised November 2024. Available at: https://www.nice.org.uk/guidance/ng245. Accessed: March 2025
- Easyhaler® Budesonide 100, 200, and 400 mcg. SmPC. Orion Pharma.
- Formoterol Easyhaler® 12 mcg. SmPC. Orion Pharma.
- Fobumix Easyhaler® 80/4.5, 160/4.5, and 320/9 mcg. SmPC. Orion Pharma.
- Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract, May 2007, 61, 5, 725–736.
- RCP. Why asthma still kills. National Review of Asthma Deaths (NRAD). May 2014. accessed 6th November 2024 from: https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills
- Geddes, D. M. (1992). Inhaled corticosteroids: benefits and risks. Thorax, 47(6), 404.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
April 2025 / RESP-330bbe(2)


