Easyhaler® Beclometasone (beclometasone dipropionate)
Product info | 12/10/2023
FULL PRESCRIBING INFORMATION CAN BE FOUND HERE

PRESENTATIONS:
Beclometasone dipropionate 200mcg
THERAPEUTIC INDICATION:
For treatment of mild, moderate, and severe persistent asthma in adults.
CONTRAINDICATIONS:
Hypersensitivity to beclometasone dipropionate, Lactose monohydrate (which contains small amounts of milk proteins). Special care in patients with active or quiescent pulmonary tuberculosis.
UNDESIRABLE EFFECTS:
Very common: Oropharyngeal Candidiasis of the mouth and throat.
Common: Hoarseness, throat irritation, cough.
Prescribers should consult the SmPC to special warnings and precautions.

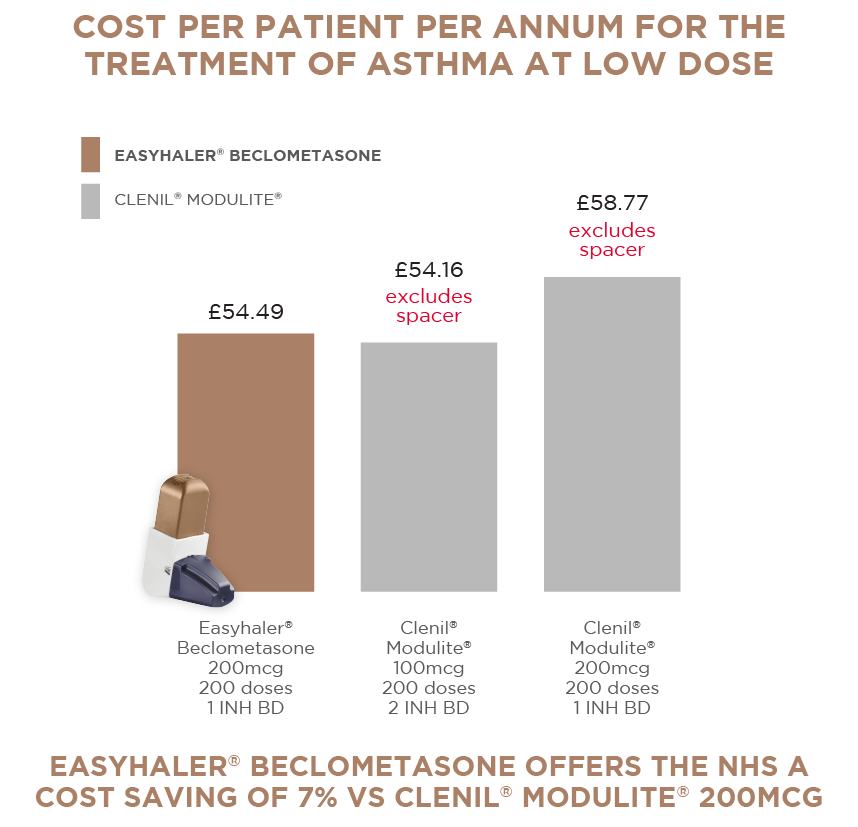
WITH EASYHALER® BECLOMETASONE1 :


EASYHALER® BECLOMETASONE is the ONLY Dry Powder Inhaler (DPI) Beclometasone available to the NHS and part of a carbon neutral inhaler range. Easyhaler® Beclometasone can help you achieve environmental targets by reducing your MDI prescribing.
|
ACHIEVE YOUR ENVIRONMENTAL TARGETS BY CHANGING YOUR PATIENTS TO EASYHALER® BECLOMETASONE.
Orion Pharma (UK) Ltd have developed this Sustainable Impact Model to support your healthcare organisation to estimate the costs and carbon impact of changing from high global warming potential inhalers to an appropriate Easyhaler® device.
|

Would your patients prefer the same device, two products, ONLY one technique?
Using the same inhaler device can offer convenience to patients, as it reduces the need to learn and manage multiple techniques and devices.
Patient education and clear instructions on using the device correctly are crucial to ensure effective and safe medication administration.
✔ Reducing MDI prescribing should be done thoughtfully and in consultation with the patient’s specific healthcare needs and preferences. The goal is to minimise the environmental impact while ensuring optimal patient care.
✔ Prescribe a range of drug therapies within the Easyhaler® range and have the flexibility to move patients across the BTS/SIGN guidelines to find and maintain the lowest controlling therapy2
ESTIMATED CARBON FOOTPRINT OF DPIs and MDIs (per 200 actuations)3
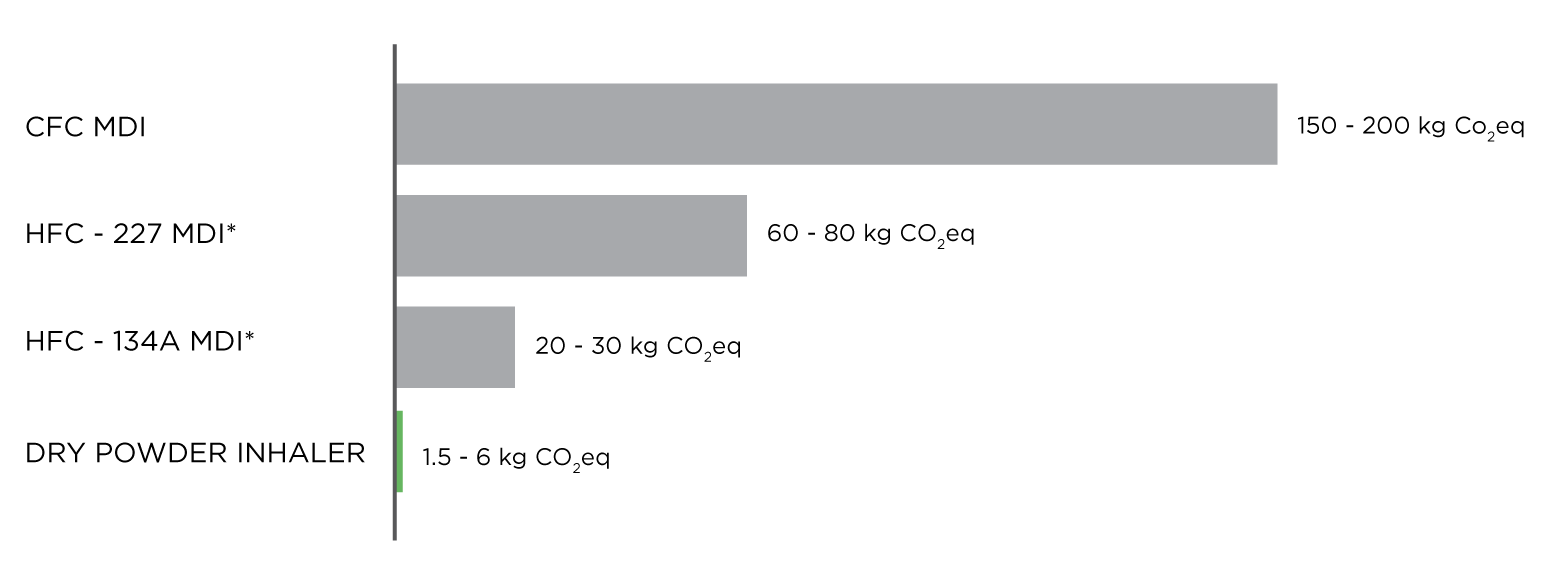
* Numbers refer to class of hydrofluorocarbon (HFC) refrigerant
Figure 1: Estimated carbon footprint of DPIs and MDIs (per 200 actuations) adapted from: Montreal protocol on substances that deplete the ozone layer. Medical and chemicals technical options committee. Assessment report 2018
BTS / SIGN = British Thoracic Society / Scottish Intercollegiate Guidelines Network, ICS = Inhaled Corticosteroids, DPI = Dry Powder Inhaler, MDI = Metered Dose Inhalers.
SmPC = Summary of Product Characteristics
Was this article helpful?
References
- Easyhaler Beclometasone 200mcg. SmPC. Orion Pharma.
- BTS/SIGN. British guidelines on the Management of Asthma - a national clinical guideline. SIGN 158 Revised July 2019. https://www.britthoracic.org.uk/qualityimprovement/guidelines/asthma/
- Montreal protocol on substances that deplete the ozone layer. Medical and chemicals technical options committee. Assessment report 2018.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
October 2023 / RESP-764(1)a



