Fusacomb Easyhaler® (salmeterol xinafoate / fluticasone propionate)
Product info | 19/12/2022

FUSACOMB EASYHALER®
Prescribing information available here
FUSACOMB EASYHALER®
● Salmeterol xinafoate/fluticasone propionate
● Strengths: 50/250 mcg and 50/500 mcg
Age
ASTHMA:
For adults and adolescents aged 12 years and older.
- Fusacomb Easyhaler® 50 / 250mcgs
- Fusacomb Easyhaler® 50 / 500mcgs
COPD:
- Fusacomb Easyhaler® 50 / 500mcgs
(Fusacomb Easyhaler® 50 / 250mcgs is not indicated for use in COPD)
Asthma
Fusacomb Easyhaler® is indicated in the regular treatment of asthma where use of a combination product (long-acting β2 agonist and inhaled corticosteroid) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short-acting β2 agonist
or
- patients already adequately controlled on both inhaled corticosteroid and long acting β2 agonist.
Chronic Obstructive Pulmonary Disease (COPD)
Fusacomb Easyhaler® 50/500mcg is indicated for the symptomatic treatment of patients with COPD, with a
FEV1 <60% predicted normal (pre-bronchodilator) and a history of repeated exacerbations, who have significant symptoms despite regular bronchodilator therapy.
SALMETEROL/FLUTICASONE COMBINATION IN AN EASYHALER® DEVICE
The efficacy and safety of Fusacomb Easyhaler has been studied and proven bioequivalent (Figure 1) to the originator product.1–3
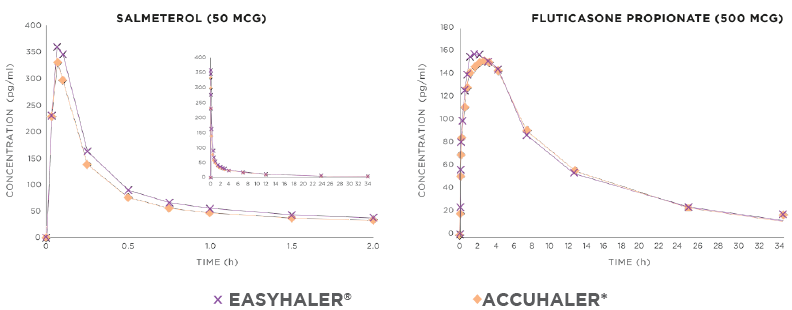
Figure 1. Fusacomb Easyhaler® is bioequivalent to the originator product.
Concentration-time curves after study treatment administrations (2x 50/500 mcg) for salmeterol (N = 59 healthy volunteers) and fluticasone (N = 61).2 Time (hours) in the x-axis = 0–2 hours (t = 0–34 hours in a small figure).
CONSISTENT DOSING ACROSS VARYING PATIENT INHALATION FLOW RATES WITH FUSACOMB EASYHALER®
Fusacomb Easyhaler® 50/500mcg provides consistent dosing across a range of inhalation flow rates typical of patients with asthma or COPD (Figure 2). The lung deposition characteristics of Fusacomb Easyhaler® – flow rate dependence of delivered dose and fine particle dose – have been proven similar to the originator product.3
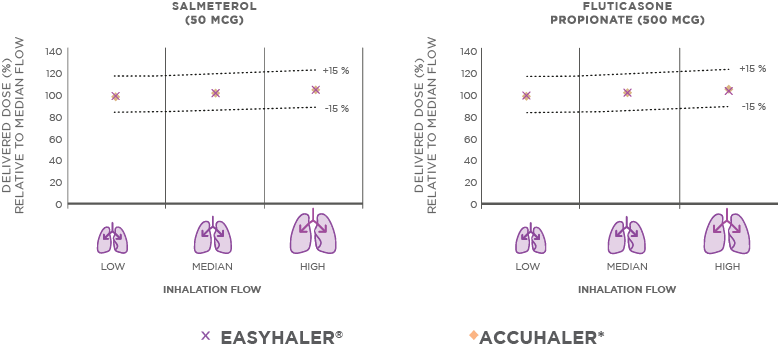
Figure 2. Fusacomb Easyhaler® offers consistent dosing across asthma and COPD patient inhalation flow rates.3
*This article refers to UK brand names. Within the clinical papers the brand names reflect the location of where the studies took place
Was this article helpful?
Read More:
Orion is a globally operating Finnish pharmaceutical company - a builder of well-being for more than 100 years. Orion develops, manufactures and markets human and veterinary pharmaceuticals and active pharmaceutical ingredients. Respiratory diseases are one of Orion’s core therapy areas. Orion's net sales in 2018 amounted to EUR 977 million and the company had about 3,200 employees. Orion's A and B shares are listed on Nasdaq Helsinki.®
You can download the Orion Sustainability Report 2021 here
As a forward-looking pharmaceutical company, Orion continues to invest in research and development of treatment options for people with asthma and COPD. The focus is on safety and quality in each step of the product life cycle while taking care of the environment. Sustainability is entwined in the whole process from R&D through manufacturing. It is also required of Orion’s providers. Orion is committed to keeping the best possible control of the environmental impacts of their own factories by reducing energy consumption and the impact of their waste waters, among others, and is making good progress in that regard. Orion works to ensure that suppliers have procedures in place to control and reduce their own environmental impacts as well. All aspects of sustainability - social, economic and environmental - are carefully considered in the whole product life cycle, including patient use and the disposal of old inhalers.
REFERENCES:
- Salmeterol/fluticasone Easyhaler® 50/250 and 50/500 mcg. SmPC. Orion Pharma.
- Kirjavainen M, Mattila L, Vahteristo M, Korhonen J, Lähelmä S. Pharmacokinetics of salmeterol and fluticasone delivered in combination via Easyhaler and Diskus dry powder inhalers in healthy subjects. J Aerosol Med Pulm Drug Deliv 2018;31:1–8.
- Jögi R, Lähelmä S, Vahteristo M, Happonen A, Haikarainen J. In vitro flow rate dependency of delivered dose and fine particle dose of salmeterol/fluticasone propionate Easyhaler® and Seretide Diskus® with patient flow rates collected in a randomized controlled trial. J Aerosol Med Pulm Drug Deliv 2018 In press.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
October 2022 / RESP-330bbb(2)




