Clinical Effectiveness of Budesonide/Formoterol Fumarate Easyhaler® for Patients with Poorly Controlled Obstructive Airway Disease: a Real-World Study of Patient-Reported Outcomes
Publikationer | 2024-01-19Study Summary:
Clinical Effectiveness of Budesonide/Formoterol Fumarate Easyhaler® for Patients with Poorly Controlled Obstructive Airway Disease: a Real-World Study of Patient-Reported Outcomes
Tamási et al. 2018. Adv Ther
- The clinical effectiveness and patient satisfaction of budesonide/formoterol dry powder inhaler Bufomix Easyhaler® was evaluated in patients with asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap (ACO) in daily clinical practice.
- The use of Bufomix Easyhaler® significantly improved disease control, quality of life, and lung function in all patient groups (P≤0.002).
- Patients were satisfied with the use of Bufomix Easyhaler®, and most patients learned to use it in less than 5 minutes.
Inhalation is the most recommended route for administering medication for asthma and COPD patients. Therefore, inhalation technique and inhaler device are important factors in achieving an effective clinical response. Here, the effectiveness of Bufomix Easyhaler® was assessed in a 12-week real-world, multicenter, open-label, non-randomized, non-interventional study among 1498 patients with asthma, COPD, or ACO in Hungary. One-third (30.4%) of the patients were newly diagnosed and 69.6% were switched to Easyhaler from other inhalers, most commonly from metered dose inhaler (MDI) (24%), Turbuhaler® (19%), or Diskus® (14.5%).
Clinical effectiveness in patients with asthma or ACO (N=720) was evaluated based on disease control (Asthma Control Test, ACT), quality of life (mini-Asthma Quality of Life Questionnaire, mini-AQLQ), and lung function (forced expiratory volume in 1s, FEV1% predicted measurement). Similarly, in the COPD and ACO patient group (N=877), disease control was evaluated by COPD assessment test (CAT), quality of life based on the modified Medical Research Council (mMRC) dyspnea scale, and lung function by FEV1% predicted measurements. Satisfaction among patients who switched from other inhalers (N=1043) was recorded by a questionnaire in a scale from 1 (very good) to 6 (unsatisfying).
The results showed that the use of Bufomix Easyhaler significantly improved disease control as demonstrated by the changes in the ACT score (from 14.2 to 21.0, P<0.001) and CAT score (from 24.2 to 18.2, P<0.001) (Figure 1). Treatment with Bufomix Easyhaler also improved the quality of life in all patient groups (P<0.001). In addition, the use of Bufomix Easyhaler resulted in significant improvement in lung function across all patient groups (P<0.001), and importantly, also in patients who were switched from previous inhalers (P<0.001).
After 12 weeks of treatment with Bufomix Easyhaler, the majority (87.2%) of asthma patients were able to reduce the use of a reliever inhaler. More than 90% of physicians considered Bufomix Easyhaler as very easy or easy to teach and use, and most patients (73.8%) learned the technique in less than 5 minutes. Notably, 72.4% of the patients rated Bufomix Easyhaler as “very good”, whereas corresponding results for MDI, Turbuhaler, and Diskus were 12.7%, 17.3%, and 18%, respectively.
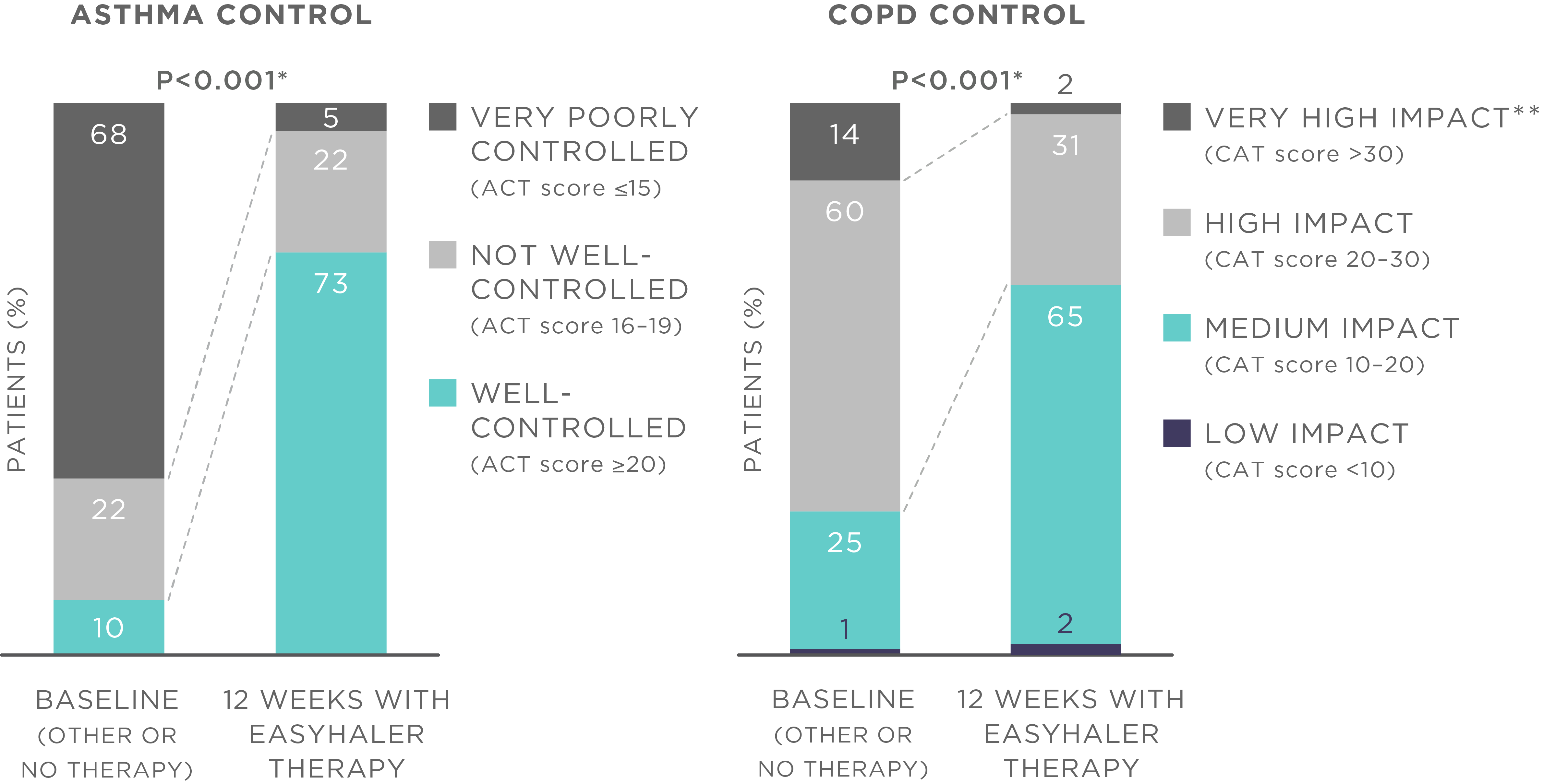
Figure 1. Bufomix Easyhaler therapy improves disease control among patients with asthma, COPD, and ACO. N (total) = 1498 patients (621 with asthma, 778 with COPD, and 99 with ACO; 455 newly diagnosed patients and 1043 patients switching from another inhaler device). *P<0.001 for average ACT and CAT score, **Impact of COPD symptoms on everyday life. Patients received 12 weeks of budesonide/formoterol Bufomix Easyhaler therapy.
This real-life study showed that Bufomix Easyhaler significantly improved disease control, quality of life and lung function among patients with asthma, COPD, and ACO. The inhaler was considered easy to teach and use, and the majority of patients rated the user satisfaction of Bufomix Easyhaler with the highest grade.
Minimiinformation
Bufomix Easyhaler® (budesonid/formoterol) [Rx] F. Astma: Inhalationspulver i styrkorna 80/4,5 mikrogram, 160/4,5 mikrogram och 320/9 mikrogram. För behandling av astma när kombinationsbehandling är lämplig. Bufomix Easyhaler är inte avsett som initial astmabehandling. Bufomix Easyhaler 80/4,5 mikrogram är godkänd från 6 år vid underhållsbehandling och från 12 år för underhållsbehandling och vidbehovsbehandling. Bufomix Easyhaler 160/4,5 mikrogram är godkänd från 12 år vid underhållsbehandling och för vidbehovsbehandling. Bufomix Easyhaler 320/9 mikrogram är godkänd från 12 år vid underhållsbehandling. Vid anfallskupering ska en snabbverkande bronkdilaterare användas. KOL: Inhalationspulver i styrkorna 160/4,5 mikrogram och 320/9 mikrogram. För symtomatisk behandling av patienter med svår KOL (FEV1 <70% av förväntat värde) och tidigare upprepade exacerbationer och som har signifikanta symtom trots regelbunden behandling med bronkdilaterare. Bufomix Easyhaler 160/4,5 mikrogram och 320/9 mikrogram är godkänd från 18 år. Senaste översyn av produktresuméer: 2022-10-21. För priser och ytterligare information se www.fass.se.

