Budesonide/Formoterol Easyhaler®: performance under simulated real-life conditions
Publikationer | 2023-08-30Study summary:
Budesonide/formoterol Easyhaler®: performance under simulated real-life conditions.
Haikarainen et al. 2017. Pulm Ther
-
The in vitro performance of the budesonide/formoterol fumarate dry powder inhaler Bufomix Easyhaler® was compared to the Symbicort® Turbuhaler® in simulated real-life conditions.
-
Easyhaler showed significantly higher consistency in drug dosing than Turbuhaler, across patient inhalation flow rates (p<0.001).
-
Easyhaler maintained its consistent performance also under environmental stress conditions, including moisture, dropping, vibration and repeated freeze-thaw cycles.
Successful treatment of patients with asthma and COPD necessitates consistent, precise and predictable performance of an inhaler – from the first dose to the last. The clinical efficacy of an inhaler should be independent of the inspiratory effort produced by the patient. In addition, the device should maintain its performance even when exposed to environmental stress conditions, such as variations in temperature or dropping. The study compared the uniformity of drug delivery characteristics of two budesonide/formoterol furamarate combination DPIs, Bufomix Easyhaler® and Symbicort® Turbuhaler®.
The consistency of delivered dose (DD) was studied for both Easyhaler and Turbuhaler at three different flow rates corresponding to pre-determined patient inhalation flows rates: low (10th percentile), median (50th percentile), and high (90th percentile). Each flow rate was tested with six devices of both products (160/4.5 µg strength), taking three devices from two batches. This study also assessed the consistency of DD and fine particle dose (FPD) through the Bufomix Easyhaler lifespan, for all three strengths available (80/4.5, 160/4.5 and 320/9 µg per inhalation). In addition, Easyhaler was exposed to various stress tests, including moisture, dropping, vibration and repeated freezing/thawing cycles. DD and FPD released from all tested inhalators were analyzed by high-performance liquid chromatography (HPLC).
The results of the study indicated that the dosing accuracy of Bufomix Easyhaler was significantly higher than the dosing accuracy of Symbicort Turbuhaler, at all three flow rates, in administering both budesonide and formoterol (Figure 1; p<0.001). DD and FPD were also demonstrated to remain consistent throughout the Easyhaler lifespan. Environmental stress conditions did not affect the DD or FPD of budesonide or formoterol released from Easyhaler.
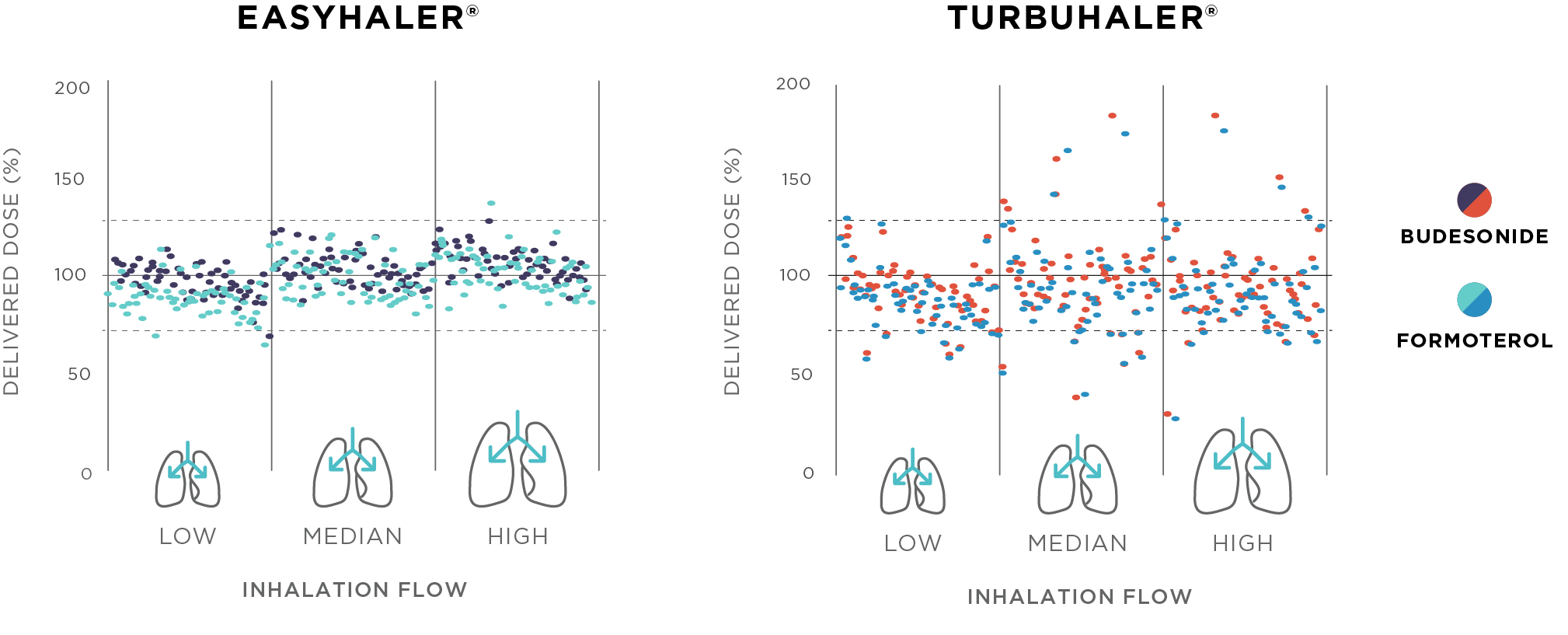
Figure 1. Dose delivery (DD) of budesonide and formoterol fumarate (strength 160/4.5 µg) from two dry powder multi-dose inhalers – Bufomix Easyhaler® and Symbicort® Turbuhaler® – at three different flow rates. The DD is shown as percent of the nominal labelled dose; each dot represents a single dose.
This in vitro study demonstrated that Bufomix Easyhaler provides consistently accurate dose delivery across patient inhalation flow rates. Easyhaler delivers drug doses accurately throughout the lifespan of the device. In addition, Easyhaler's performance remains stable in simulated real-life conditions. Thus, Easyhaler is a robust and reliable DPI for the maintenance of asthma and COPD.
Learn more about the study results by watching the animation:
Minimiinformation
Bufomix Easyhaler® (budesonid/formoterol) [Rx] F. Astma: Inhalationspulver i styrkorna 80/4,5 mikrogram, 160/4,5 mikrogram och 320/9 mikrogram. För behandling av astma när kombinationsbehandling är lämplig. Bufomix Easyhaler är inte avsett som initial astmabehandling. Bufomix Easyhaler 80/4,5 mikrogram är godkänd från 6 år vid underhållsbehandling och från 12 år för underhållsbehandling och vidbehovsbehandling. Bufomix Easyhaler 160/4,5 mikrogram är godkänd från 12 år vid underhållsbehandling och för vidbehovsbehandling. Bufomix Easyhaler 320/9 mikrogram är godkänd från 12 år vid underhållsbehandling. Vid anfallskupering ska en snabbverkande bronkdilaterare användas. KOL: Inhalationspulver i styrkorna 160/4,5 mikrogram och 320/9 mikrogram. För symtomatisk behandling av patienter med svår KOL (FEV1 <70% av förväntat värde) och tidigare upprepade exacerbationer och som har signifikanta symtom trots regelbunden behandling med bronkdilaterare. Bufomix Easyhaler 160/4,5 mikrogram och 320/9 mikrogram är godkänd från 18 år. Senaste översyn av produktresuméer: 2022-10-21. För priser och ytterligare information se www.fass.se.

